Global Rating Scale (GRS)
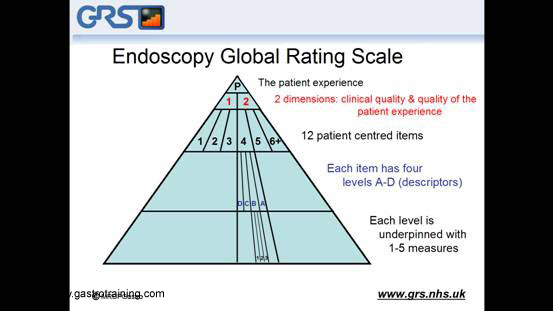
The GRS is a tool that enables endoscopy units to assess how well they provide a patient-centred service. It is a web-based assessment tool that makes a series of statements requiring a yes or no answer. From the answers it automatically calculates the GRS scores, which provides a summary view of your service. The scale tries to strike a balance between being comprehensive but not too complicated. To achieve this, the scale has different layers:
1. Patient experience is divided into two Dimensions:
- Clinical quality and
- Quality of the patient experience.
2. Each dimension is divided into six ( so a total of 12) patient centred items
Clinical quality
- Appropriateness
- Information/consent
- Safety
- Comfort
- Quality
- Timely results
Quality of the patient experience
- Equality
- Timeliness
- Choice
- Privacy and dignity
- Aftercare
- Ability to provide feedback
3. Each item is divided into fourDescriptors or levels from D,C,B,A
- D: Basic to
- A: Excellent
4. Each descriptor is then underpinned by 1-5 Measures: which are unambiguous statement and you have to provide yes or no answer and then it will give you your GRS score
Below is an example of the levels and the measures: we used the example of Dimension of clinical quality and item of information/consent
Clinical Quality
| Consent Process Including Patient Information | |||
| 1.1 | There is a published patient information sheet for all diagnostic procedures performed in the department | Level D | |
| 1.2 | The Trust policy for consent is available in the Department in written and electronic form | ||
| 1.3 | There is a published patient information sheet for all endoscopy procedures performed in the department | Level C | |
| 1.4 | All patients are given an opportunity to ask questions about the procedure prior to the endoscopy by a professional trained in the consent process | ||
| 1.5 | Signatures are obtained on a consent form for all patients who are able to sign the form and there are procedures in place for those who cannot sign | ||
| 1.6 | All patients are given sufficient time to ask questions before entering the procedure room | Level B | |
| 1.7 | All consent signatures are obtained outside the procedure room. | ||
| 1.8 | There is written guidance within the department for withdrawal of consent during an endoscopic procedure | ||
| 1.9 | All published patient information sheets are reviewed annually and changed as necessary. | Level A | |
| 1.10 | Patients’ frequently asked questions are incorporated into the patient information sheets | ||
| 1.11 | There is at least one annual survey of patients’ experience of consent for endoscopic procedures | ||
| 1.12 | Findings of the survey are acted upon within a three-months of its completion | ||
| 1.13 | Failure to comply with withdrawal of consent guidance is registered as an adverse clinical incident | ||
References/Acknowledgement:








