Wireless Capsule Endoscopy (WCE)
Wireless capsule endoscopy using PillCam SB capsule was approved by the U.S. Food and Drug Administration in 2001. It was the first digital imaging device for the small intestine that provided direct high-resolution images of the mucosa.
Advantages:
- Easily ingested, painless procedure
- Progresses naturally through the GI tract via peristalsis
- Ambulatory examination
- Disposable video capsule
Discuss the components of a capsule endoscope?
It contains an imaging device and light-source on one-side and transmits images at a rate of 2 images per second generating more than 50,000 pictures over an 8-hour period.
- Optical dome
- Lens holder
- Lens
- Illuminating LEDs
- Imager
- Battery
- RF Transmitter
- Antenna
- Dimensions 27X11 mm and weighs 3.7gm
How is capsule endoscopy performed?
- A patient fasts starting at midnight the day before the procedure. Some physicians use bowel prep as for colonoscopy.
- A sensor array is attached to the patient’s abdomen and the data recorder to a belt around the patient’s waist. The capsule transmits data to the Sensor Array which is secured to the patient’s abdomen. The Sensor Array is connected to the Data Recorder (which stores the data), worn on the belt.
- The patient is then asked to swallow the capsule. The patient can resume daily activities once he or she has successfully swallowed the video capsule.
- After 8-hours the patient returns to the physician’s office to return the data recorder and the pill passes naturally with a bowel movement usually within 24 hours.
- Images are downloaded from the data recorder to the workstation for review and diagnosis.
- Patients are allowed to drink clear liquids after two hours and eat a light meal after four hours following the capsule ingestion
- It takes 30 to 60 minute reading time per study
Discuss the effectiveness of WCE over other methods of investigating small bowel?
Summary of Incremental Yield (IY) of WCE over other modalities for suspected or diagnosed Crohn’s disease.
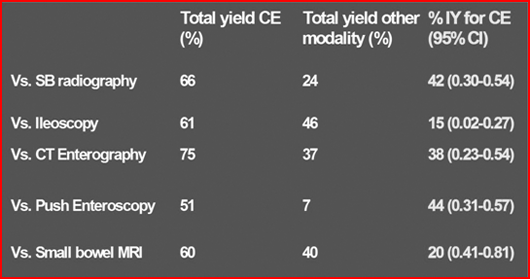
Ref: Triester SL, et al Am J Gastroenterol 2006
Discuss the indications of WCE?
- Obscure GI bleed: WCE considerably shortens the time to diagnosis and lead to definitive treatment in a relevant proportion of patients. WCE also spare a number of alternative investigations with low diagnostic yield
- Suspected Crohn’s disease
- Assessment of coeliac disease
- Screening and surveillance for polyps in familial polyposis syndromes
Discuss capsule retention?
Capsule retention is defined as having a capsule remain in the digestive tract for more than two weeks.
Retention has been reported at ~ 0.75% overall or up to 13% in patients with known strictures (ref- Signorelli C, et al. Digestive and Liver Disease 2006 )
The published incidence of capsule retention varies with the risk being related to the indication: (Ref- Lewis B, Endoscopy 2005)
- OGIB ~ 1.5%
- Known Crohn’s disease (CD) ~ 5%
- Suspected CD ~ 1.4%
- In healthy volunteers, during clinical trial, no incident of capsule retention occurred in 773 individuals
What are the causes of capsule retention?
Causes of retention cited in the literature include:
- NSAID strictures
- Crohn’s disease
- Small bowel tumors
- Radiation enteritis
- Surgical anastomotic strictures
Capsule retention has not been reported in “normal” anatomy or anatomical variants (e.g., colon or small bowel diverticulosis and appendiceal orifices).
How do you treat capsule retention?
Capsule retention is managed by medical (steroid may improve strictures), surgical, or endoscopic intervention (using a balloon enteroscope)
Can capsule retention be prevented?
- Current methods for identifying intestinal strictures lack sensitivity and specificity to evaluate GI tract patency.
- The majority of capsule retentions have occurred in patients with normal small bowel radiological studies. Conversely, results have suggested that functional patency may be present in patients with radiologically-documented strictures.
However, capsule retention can be prevented by using a patency capsule
Discuss patency capsule?
The patency capsule is a simple and convenient accessory to video capsules that is intended to verify functional patency of the GI tract in patients with known or suspected strictures prior to administration of the video capsule.
A patency capsule stays intact for minimum 30 hours post-ingestion. It subsequently disintegrates and is excreted. It emits electromagnetic waves at 64 KHz when sensing electromagnetic waves at 128 KHz.
Procedure:
Day 1: Patient swallows the patency capsule
Day 2: The patient’s abdomen is scanned using a hand held patency scanner. Patency is proven if the capsule is not detected by the patency scanner.
What are the contraindications of WCE?
- Patients with known or suspected GI obstruction, strictures or fistulas based on clinical presentation or pre-procedure testing
- Patients with cardiac pacemakers or other implanted electro-medical devices
- Patients with swallowing disorders